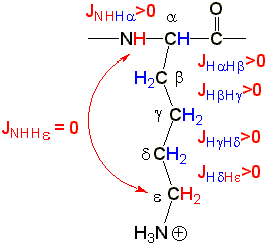
NH->H(a), H(a)->H(b), H(b)->H(g), H(g)->H(d), H(d)->H(e)
form an uninterrupted J>0 pattern which can be used by (G)TOCSY
| GTOCSY.help | helpfile for GTOCSY experiments (parameter: mix) |
(G)TOCSY, or (Gradient-enhanced) TOtal Correlation SpectroscopY, is a two-dimensional homonuclear technique providing correlations between protons that are "connected" through an uninterrupted pattern of coupling constants. It is NOT based on long-range coupling constants.
Examples:
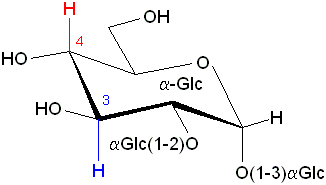
| The H1 and H4 protons of a pyranose ring are not coupled (JH1H4=0) but there is an uninterrupted pattern of J>0: H1->H2, H2->H3, H3->H4 |
|
| NH and H(e) of the amino acid
Lysine (Lys, K) are not coupled but NH->H(a), H(a)->H(b), H(b)->H(g), H(g)->H(d), H(d)->H(e) form an uninterrupted J>0 pattern which can be used by (G)TOCSY |
|
GTOCSY and TOCSY are fundamentally the same techniques. The gradient version provides cleaner spectra with more flexibility in the choice of the number of transients (nt). GTOCSY is implemented on all our spectrometers.
Some of the features of GTOCSY:
| + | almost as sensitive as GCOSY |
| + | "as many connectivities as desired" by changing one single parameter in the same pulse sequence (see below) |
| + | analysis does not require crossing of the diagonal (the main advantage over GCOSY) |
| - | does not remove solvent signal (usually not a problem in common solvents including D2O, exception: H2O) |
| - | sometimes GTOCSY works so well that, at least for carbohydrates, it is difficult to distinguish e.g. an H1->H2 from an H1->H3 correlation without additional GCOSY information; this is much less a problem for amino acids where the chemical shift usually makes it clear what type of side-chain proton is responsible for a correlation |
By calling in a standard parameter set (EZ NMR: GTOCSY button in group 8: Select Technique), the user does not have to worry about parameter settings with one possible exception: the mixing time 'mix', also known as spin-lock time.
The length of mix (in seconds) determines how far away in the spin system correlations can be observed:
1
mix = SUM ------
10 J
Very short values for mix produce in essence a GCOSY spectrum. Long values can provide correlations all the way from H1 to H6a and H6b in pyranose rings or from NH to H(e) in Lys.
For amino acids, 0.080 sec is sufficient to get correlations from NH to the protons at the end of even the longest side chain (Lys).
Approximation of required mix [sec] for various pyranose systems:
| pyranose | H1->H2 | H1->H3 | H1->H4 | H1->H5 | H1->H6 |
| β-Glc (NAc) | 0.015 | 0.030 | 0.045 | 0.060 | 0.080 |
| β-Gal (NAc) | 0.015 | 0.030 | 0.060 | 0.150 | 0.170 |
| α-Glc (NAc) | 0.030 | 0.045 | 0.060 | 0.075 | 0.095 |
| α-Gal (NAc) | 0.030 | 0.045 | 0.075 | 0.160 | 0.180 |
| α-Fuc | 0.030 | 0.045 | 0.075 | 0.160 | 0.180 |
| α-Man | 0.070 | 0.110 | 0.120 | 0.140 | 0.160 |
| β-Man | 0.130 | 0.160 | 0.180 | 0.190 | 0.210 |
These are theoretical values; often 10 to 20% more is needed to achieve the desired "walk" around the spin system.
Note that not all
long values for mix can be used
(hardware protection). The limit depends
on the power level
(slpwr) for the spin-lock. The maximum for mix is ca. 0.3 at power levels below 40 and 0.15 sec at power
levels above 40.
An additional consideration is that T2 relaxation reduces the signal intensity with
increasing length of mix. Consequently, a compromise may have to be found, especially for
molecules with broad lines (short T2).