|
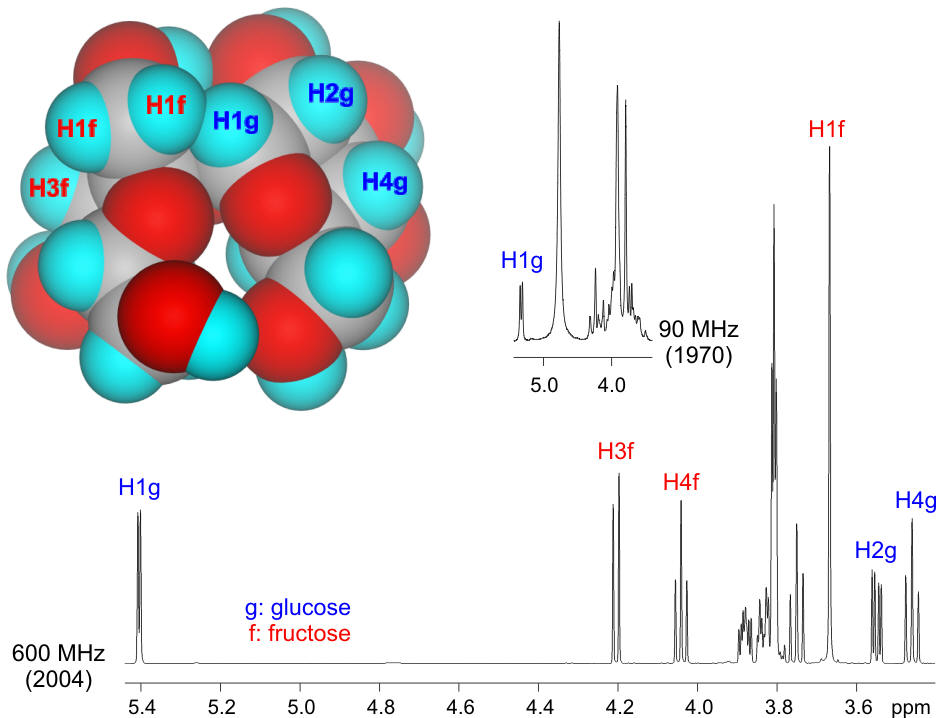
Professor Ray Lemieux Professor Lemieux was the first scientist to chemically synthesize sucrose, more commonly known as table sugar. This achievement in 1953 caused the commodities market to dip until it was realized that only 34 milligrams of material had been made. Many years latter, in 1982, he was the first to use nuclear magnetic resonance spectroscopy (NMR) to deduce the three dimensional structure of the sucrose molecule and to postulate a hydrogen bond between one hydroxyl group of the fructose ring and one hydroxyl group of the glucose ring. Sucrose is shown below as a space filling representation where each carbon, oxygen and hydrogen atom is represented by spheres. Sucrose is a disaccharide composed of the two monosaccharides, glucose and fructose. The glucose unit exists as a ring containing six atoms while the fructose ring contains five atoms. Nuclear magnetic resonance spectroscopy, in its simplest form, allows us to observe a signal for each hydrogen atom of a molecule. Since these are at the periphery of the molecule they are very useful handles to sense which parts of a molecule are in contact with each other. The nuclear magnetic spectrum consists of peaks, each with a characteristic position that reflects the chemical environment of the hydrogen atoms. The separation of peaks is quite small but increases in proportion to the magnetic field strength. In the mid 1950s, when the first NMR spectrometers were in use, their magnets operated at 40 and 60 MHz. Neither of these are used today in research labs. A 90 MHz NMR spectrum is shown here together with one of the modern NMR spectra recorded at 600 MHz (ten times stronger than those first used by Professor Lemieux). The same relative scale is used for each spectrum and shows the significantly increased dispersion of the signals at 600 MHz which allows us to determine three-dimensional structures. In the illustration below, hydrogen atoms belonging to the glucose component of the sucrose molecule are correlated with the peak for this atom in the NMR spectrum, for example H1g seen as the left most peak. Similarly some hydrogen atoms for the fructose component of sucrose are also identified. Because the molecule is a solid object some of the hydrogen atoms that are labelled in the spectrum of sucrose cannot be seen; they are located on the opposite side of the molecule. A subtlety of the NMR spectrum is that some NMR peaks appear as multiplets, i.e. peaks with more than one “spike”. This feature carries important structural information about the number of hydrogen atoms attached to neighbouring carbon atoms. The spacing between the components of the multiplets tells us about their relative orientation to each other. Professor Lemieux pioneered the deduction of the principles that apply to this angular relationship and was the first to correlate such features with three-dimensional properties of a molecule. |
