Department of Chemistry,
University of Alberta
September 2005
NMR News 2005-03
News and tips from the NMR support group for users of the Varian NMR systems in the
Department
Editor: Albin.Otter@ualberta.ca
http://nmr.chem.ualberta.ca
There are no fixed publishing dates for this newsletter; its appearance solely depends on whether there is a need to present information to the users of the spectrometers or not.
Other content of this NMR News is no longer meaningful and has been removed May 2010.
Contents
|
|
Solvents
Representative spectra of seven
solvents are shown below:
CDCl3, C6D6, CD2Cl2,
D2O, CD3OD, DMSO,
Acetone-D6. The samples used to measure the
spectra contained only the solvent (plus impurities). The solvents were
stored with molecular sieves in the bottle for at least one night.
Recorded at 400 MHz (100MHz C13),
except where indicated. A systematic overview of the coupling patterns
is shown here. |
|
CDCl3: 7.26 ppm (H1) |
The H2O impurity is at 1.527 ppm.
1J(CH) = 209.0 Hz, the lines close to the solvent peak are |
|
CDCl3: 77.0 ppm (13C) |
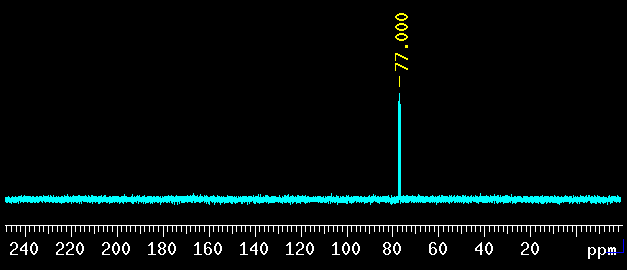
The 1J(CD) coupling constant is 32.0 Hz. Intensity is 1:1:1 because D is a spin=1 nucleus and each carbon is coupled to one D. |
| C6D6: 7.15 ppm (H1) |
The H2O impurity is at 0.388 ppm.
1J(CH) = 158.0 Hz. |
| C6D6: 128.0 ppm (13C) |
The 1J(CD) coupling constant is 24.3
Hz. Intensity is 1:1:1 because D is a spin=1 nucleus and |
| CD2Cl2: 5.32 ppm (H1) |
|
The H2O impurity is at
1.513 ppm.
1J(CH) = 178.0 Hz (not visible here). 2J(HD) = 1.1 Hz. |
|
CD2Cl2: 53.8 ppm (13C) |
|
The 1J(CD) coupling
constant is 27.3
Hz. Intensity is 1:2:3:2:1 because D is a spin=1 nucleus and each carbon is
coupled to two D. |
| D2O: 4.75 ppm (H1) |
|
Typically free of impurities but the residual HDO peak is quite intense.
The C13 spectrum is nothing but
baseline, of course! |
| CD3OD: 3.30 ppm (H1) |
|
The CD3-OH and
H2O resonance are basically identical at 4.8 ppm. 2J(HD) = 1.65 Hz.
Same spectrum as right
above but 40 times enlarged vertically. 1J(CH) = 140.0 Hz.
Interesting is the small signal at 3.32 ppm. It arises from CH2D-OD
and the 2J(HD) is also 1.65 Hz. Intensity is 1:1:1 because only one D
is present to split the two protons! The 0.02 ppm
difference in chemical shift is a so-called isotope
shift. The rest is due to spinning side bands (ssb). |
|
CD3OD: 49.0 ppm (13C) |
|
The 1J(CD) coupling
constant is 21.4
Hz. Intensity is 1:3:6:7:6:3:1 (at least in theory) because D is a spin=1 nucleus and
the carbon
is coupled to three D. |
| DMSO: 2.49 ppm (H1) |
|
The H2O impurity is at
3.3 ppm (and quite a lot of it which is typical for DMSO, even when stored
|
|
DMSO: 39.5 ppm (13C) |
|
The 1J(CD) coupling
constant is 21.0 Hz. Intensity is 1:3:6:7:6:3:1 because D is a spin=1 nucleus and each carbon
is coupled to three D. |
| Acetone-D6: 2.04 ppm (H1) |
|
The H2O impurity is at
2.79 ppm in this case but the chemical shift is strongly
concentration-dependent.
A closer look shows that there are two
water peaks: the singlet at 2.79 ppm is from H2O, the
2J(HD) = 2.2 Hz.
Intensity is 1:2:3:2:1 because D is a spin=1 nucleus and the H in CHD2-CO-CD3
Same spectrum as right
above but 20 times enlarged vertically and recorded at 600 MHz. 1J(CH) = 126.0 Hz. 2J(HD) =
2.2 Hz.
Interesting are the small signals at ca. 2.06 and 2.07 ppm. 2.06 ppm is from CH2D-CO-CD3
and its 2J(HD) is also 2.2 Hz. Intensity is 1:1:1 because only one D
is present to split the two protons! |
|
Acetone-D6: 29.8 and 206.1 ppm (13C) |
|
The 1J(CD) coupling
constant is 19.4 Hz. Intensity should be 1:3:6:7:6:3:1 because D is a spin=1 nucleus and each carbon is
coupled to three D. There is some distortion present in the
intensities. In addition a strong singlet at 206.3 ppm is observed from the
carbonyl group. |
| The Table below provides an overview of the
coupling patterns based on one spin 1/2 nucleus (for example H1, 13C and many
more) and zero, one, two and three deuterium atoms coupled to the spin 1/2
nucleus by the same J(HD) or J(CD) coupling constant. Intensities of the
observed signals are shown with color coding to indicate the origin of each
line's intensity. Dashed signals are only shown to indicate the origin of the
coupling pattern but do not contribute to the final intensity directly (but
indirect through the subsequent lines). Coupled nuclei are shown in bold purple color. |
|
H1 or C13 spin=1/2 |
D spin=1 |
coupling patterns |
examples |
| 1 | 0 |
|
CHCl3 in CDCl3 |
| 1 | 1 |
|
C13-D: H1-D: |
|
1 |
2 |
|
C13-D:
H1-D: |
| 1 | 3 | 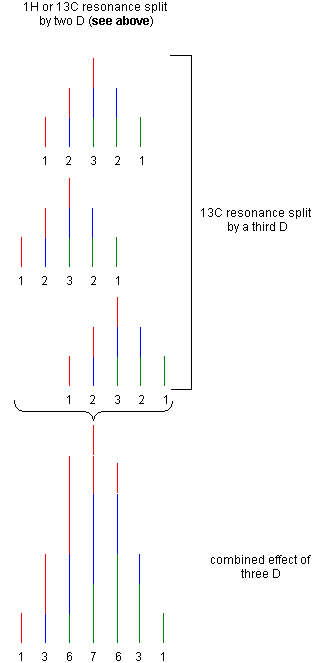 |
C13-D:
CD3 in: |
|
H1 or C13 spin=1/2 |
D spin=1 |
coupling patterns |
examples |